Reproduce Laboratory
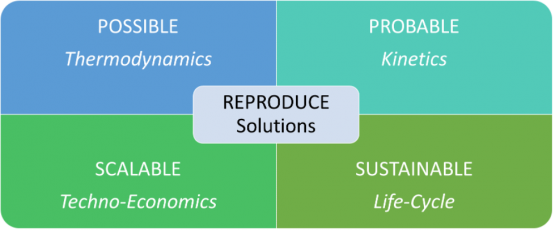
The REPRODUCE – REmediation and PRODuction Using Computational and Electro(chemical) techniques – Laboratory focuses on the application of engineering principles to approach global challenges related to waste and consumer needs. Research in the laboratory employs the use of computational chemistry, process simulations, chemical and electrochemical driving forces and material science in the development of technologies, products and methods. Overall, these complementary approaches ensure the generation of solutions that are possible via thermodynamics, probable via kinetics, scalable via techno-economic analyses and sustainable via life-cycle analyses. The laboratory is housed within the Institute for Sustainable Energy and the Environment at Ohio University.
Laboratory Updates
-
September 2022: Dr. Daramola will be giving an invited seminar on the 28th at Georgia Institute of Technology on "Modular Resource Recovery Facilities explored via Electrochemical Nutrient Reduction from Wastewater"
-
July 2022: Three abstracts submitted for the 2022 AIChE Annual Meeting in Phoenix Arizona have been accepted for presentation. Congratulations to Ajayi and Heydarian, who are first-time attendees and Ojoawo, who is a second-time attendee.
-
Bench-Scale Testing of Electrochemical Recovery of Phosphorus from Post-Digester Municipal Wastewater Driven By Magnesium Salt (Presenting Author: Ajayi)
-
Evaluating Transport Factors to Understand Electrochemical Nutrient Removal and Recovery from Synthetic Animal Wastewater (Presenting Author: Heydarian)
-
Statistical and Energy Consumption Analyses of Multi-Factor Effect on Nutrient Removal and Recovery Via Electrochemical Animal Waste Remediation (Presenting Author: Ojoawo)
-
-
June 2021: Dr. Daramola has been awarded a 2022 Ralph E. Powe Junior Faculty Enhancement Award by Oak Ridge Associated Universities. The award and the associated work is described in this OHIO news article.
-
December 2021: Three abstracts submitted for the 2022 American Chemical Society Spring Meeting have been accepted for presentation.
-
December 2021: Ardavan Zanganeh (Graduate Student) joins REPRODUCE Laboratory to enhance ongoing work on wastewater remediation.
-
December 2021: Lawrence Ajayi (Graduate Student) joins REPRODUCE Laboratory to enhance ongoing work on wastewater remediation.
-
November 2021: Two papers were presented at the 2021 AIChE Annual Meeting (in-person) by Chukwuka and Ojoawo. Congratulations to both first-time conference attendees.
-
October 2021: The first publication from the laboratory is published in ACS ES&T Water: Equilibrium-Based Temperature-Dependent Economic Analysis of the Recovery of Phosphorus from Different Wastewater Streams via Chemical Precipitation by Pindine, Trembly and Daramola.
-
September 2021: Drs. Daramola, Trembly and DeForest are funded by the U.S. Department of Energy for "Combined Nitrogen (N) and Phosphorous (P) Recovery via Electrochemical Technology Integration into Municipal Wastewater Treatment Facilities." This project is part of REPRODUCE Lab's wastewater portfolio.
-
September 2021: Abstract submitted for the 2021 AIChE Annual Meeting Minority Affairs Commission Poster Session was accepted: Thermal Properties of Heat-treated Coal Under Inert Conditions (Presenting Author: Chukwuka).
-
September 2021: Sana Heydarian (Graduate Student) joins REPRODUCE Laboratory to enhance ongoing work on wastewater remediation.
-
July 2021: Abstract submitted for the 2021 AIChE Annual Meeting was accepted: pH Effects on Factors Influencing Nutrient Removal and Recovery from Synthetic Animal Waste Via Chemical and Electrochemical Techniques (Presenting Author: Ojoawo).
-
May 2021: Sophia Almanza (Undergraduate Student) joins REPRODUCE Laboratory to enhance ongoing work on thermosetting polymer composites.
-
April 2021: Jessica Chukwuka (Graduate Student) joins REPRODUCE Laboratory to enhance ongoing work on thermosetting polymer composites.
-
April 2021: Dr. Daramola is published in Journal of CO2 Utilization
-
March 2021: Francesca Carney (Undergraduate Student) joins REPRODUCE Laboratory to enhance ongoing work on wastewater remediation.
-
March 2021: Abstracts submitted for the 239th Electrochemical Society Meeting were accepted: Electrochemical Animal Waste Remediation: Multi-Factor Effect Analyses on Nutrient Reduction and Struvite Deposition (Presenting Author: Ojoawo) and Thermodynamic Modeling of Phosphorus Recovery from Wastewater for Process Optimization (Presenting Author: Dildine).
-
January 2021: Sana Jamshidifard (Graduate Student) joins REPRODUCE Laboratory to enhance ongoing work on polymer upcycling.
-
January 2021: Dr. Daramola is published in ChemistryOpen.
-
December 2020: Two abstracts were submitted for the spring 2021 Electrochemical Society Meeting
-
October 2020: Dr. Daramola is published in the Journal of the Electrochemical Society.
-
August 2020: REPRODUCE Laboratory is established at Ohio University and is joined by two researchers: Babatunde Ojoawo (Graduate Student) and Garrett Dildine (Undergraduate Student) to enhance ongoing work on wastewater remediation.
Research
Research in the laboratory is currently supported by funds from Russ College of Engineering and Technology, the Ohio Water Development Authority and the Department of Energy’s Advanced Manufacturing Office.
Wastewater Remediation
Commercial and municipal activities involve constant withdrawal of water from natural sources (lakes, rivers, aquifers etc.) accompanied with constant rejection of water to treatment facilities. This effluent water contains various kinds of waste products including chemicals, urine, toxins etc., which at the molecular level are products that can be re-used in other processes after treatment. Human waste contains unused nitrogen that is useful for agricultural applications; mining waste contains metals that can be reused for renewable energy applications; food waste contains carbohydrates and proteins that can be reused for specialty chemical production etc. In essence, wastewater – once treated – can be a source of raw materials for applications and serve as a foundation for the circular economy.
The REPRODUCE Lab is currently using electrolyte modeling and electrochemical reactor design to understand and predict conditions for optimum resource recovery from municipal wastewater with minimal process chemical input.
Polymer Upcycling
Plastic materials are ubiquitous in the consumer space and significantly contribute to municipal solid waste due to the difficulties associated with re-use/recycling. The most recalcitrant species are polyethylene, polypropylene, polyvinylchloride and polystyrene: which possess highly stable C-H bonds, account for 77% of the global plastic production but only 17% of the recycling rate. Recent efforts have explored the conversion of these recycling-resistant polymers to fuels and chemicals, with chemical products potentially offering higher economic benefits to alleviate the cost of recycling.
The REPRODUCE Lab is currently developing techniques for generating non-fuel building blocks from thermoplastic polymers via a combination of physicochemical methods including thermolysis, solvolysis and electrolysis.
Thermosetting Polymers
The first synthetic polymer material invented, phenol formaldehyde (PF) aka Bakelite, was a thermoset that is still in use today in filtration, abrasive and friction applications and globally produced at 6 million tons per year. These PF resins possess high tensile and flexural strengths, large flexural moduli and high thermal stability. In addition, PF-based composites offer structural and thermal advantages, while negating the brittleness and formaldehyde emissions associated with the base PF thermoset. In addition, opportunities exist to make these resins more environmentally benign by incorporating biomass derivative (lignin) as opposed to a fossil fuel derivative (phenol).
The REPRODUCE Lab is currently developing advanced PF thermosets through the incorporation of filler materials with similar functional groups to the PF resin to strengthen bonding.
People
Principle Investigator
Ph.D. Chemical Engineering, Ohio University ‘11
B.S. Chemical Engineering, Ohio University ‘04
Graduate Students
Ardavan Zanganeh
Chemical Engineering Ph.D. Student
Joined December 2021
Lawrence Ajayi
Chemical Engineering Ph.D. Student
Joined December 2021
Sana Heydarian
Chemical Engineering Ph.D. Student
Joined September 2021
Jessica Chukwuka
Chemical Engineering Ph.D. Student
Joined April 2021
Babatunde Ojoawo
Chemical Engineering Ph.D. Student
Joined August 2020
Undergraduate Students
-
Kristen Jamora, Chemical Engineering 3rd Year, Joined May 2022
-
Sophia Almanza, Chemical Engineering 4th Year, Joined May 2021
Alumni
-
Francesca Carney, Chemical Engineering, Joined March 2021, Left December 2021 (Employed at Honeywell)
-
Garrett Dildine, Civil and Environmental Engineering, Joined August 2020, Left May 2021 (Graduate Studies at Carnegie Mellon University)
Publications
- G.P. Pindine, J.P. Trembly and D.A. Daramola, “Equilibrium-Based Temperature-Dependent Economic Analysis of the Recovery of Phosphorus from Different Wastewater Streams via Chemical Precipitation,” ACS EST Water 1, pp 2318 (2021).
- S. Velraj, D.A. Daramola and J.P. Trembly, “A novel solid oxide electrolytic cell with reduced endothermic load for CO2 electrolysis using (La0.80Sr0.20)0.95MnO3-δ cathode,” Journal of CO2 Utilization 48, pp 101527 (2021).
- E. Grossman, D.A. Daramola and G.G. Botte, “Comparing B3LYP and B97 dispersion-corrected functionals for Studying Adsorption and Vibrational Spectra in Nitrogen Reduction,” ChemistryOpen 10, pp 316 - 326 (2021). OPEN ACCESS and COVER
- Z. Belarbi, D.A. Daramola and J.P. Trembly, “Bench-Scale Demonstration and Thermodynamic Simulations of Electrochemical Nutrient Reduction in Wastewater via Recovery as Struvite,” Journal of the Electrochemical Society 167, pp 155524 (2020). OPEN ACCESS
- Y.A. Al Majali, C.T. Chirume, E.P. Marcum, D.A. Daramola, K.S. Kappagantula and J.P. Trembly, “Coal-Filler-Based Thermoplastic Composites as Construction Materials: A New Sustainable End-Use Application,” ACS Sustainable Chemistry & Engineering 7, pp 16870 – 16878 (2019).
- A. Estejab, D.A. Daramola and G.G. Botte, “Mathematical Model of a Parallel Plate Ammonia Electrolyzer for Combined Wastewater Remediation and Hydrogen Production,” Water Research 77, pp I33 – I45 (2015).
- G.G. Botte, D.A. Daramola and M. Muthuvel, “9.14 Preparative Electrochemistry for Organic Synthesis," In Comprehensive Organic Synthesis II (Second Edition), G.A., Molander; P. Knochel, Eds., Elsevier: Amsterdam, 351 – 389 (2014).
- D.A. Daramola and G.G. Botte, “Theoretical Study of Ammonia Oxidation on Platinum Clusters – Adsorption of Intermediate Nitrogen Dimer Molecules,” Journal of Colloid and Interface Science 402, pp 204 – 214 (2013).
- D.A. Daramola and G.G. Botte, “Theoretical study of ammonia oxidation on platinum clusters – Adsorption of ammonia and water fragments,” Computational and Theoretical Chemistry 989, pp 7 – 17 (2012).
- D.A. Daramola, D. Singh and G.G. Botte, “Dissociation rates of urea in the presence of NiOOH catalyst: a DFT analysis,” Journal of Physical Chemistry A 114, pp 11513 – 11521 (2010).
- D.A. Daramola, M. Muthuvel and G.G. Botte, “Density functional theory analysis of Raman frequency modes of monoclinic zirconium oxide using gaussian basis sets and isotopic substitution,” Journal of Physical Chemistry B 114, pp 9323 – 9329 (2010).